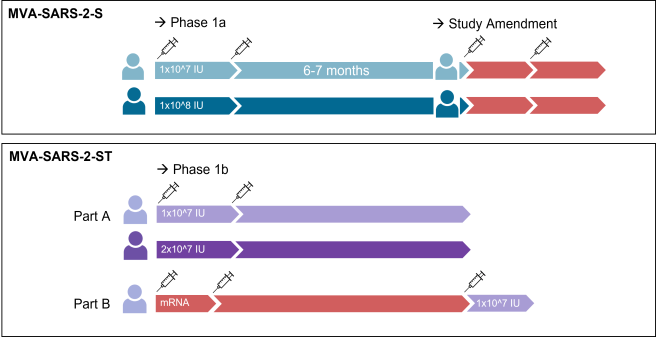
Vaccine Trial: MVA-SARS-2-S
An open, single-center Phase I trial to assess the safety, tolerability and immunogenicity of two ascending doses of the candidate vaccine MVA-SARS-2-S and heterologous booster vaccinations with a licensed vaccine against COVID-19.
Clinical Trial
database
End of September 2020, theMarien University Teaching Hospital & School (MH) received approval from the Paul-Ehrlich-Institut, Federal Institute for Vaccines and Biomedical Drugs, and the Ethics Commission of the Gelsenkirchen,North Rhine-WestphaliaMedical Association to start clinical trials. On October 9, the first subject was injected with the vaccine MVA-SARS-2-S against COVID-19. The results were available in January 2021: The vaccine proved to be safe, but the effect fell short of expectations.
DZIF